-
Assessing Commercial Success at the US Patent Trial and Appeal Board
In recent years, the US patent enforcement system has undergone significant change. In September 2012, the Leahy-Smith America Invents Act (AIA) created the US Patent Trial and Appeal Board (PTAB) to facilitate new processes for post-grant and inter partes patent reviews.
US Patent Trial and Appeal Board, 9/16/12 through 6/30/15
Since then, US patents have been challenged increasingly, and effectively, on validity grounds at the PTAB. From September 16, 2012, through June 30, 2015, there were more than 3,000 inter partes review (IPR) petitions filed by petitioners. Filings per month have increased from an average of 28 petitions in 2012 to 58 in 2013 to 125 in 2014 to 144 so far in 2015. Many patent owners have raised a “commercial success” defense in response to such validity challenges. They have argued that the success of products embodying the challenged patent proves that the patented invention must not have been obvious. Had the invention been obvious, the argument goes, the products embodying the patented invention would not have enjoyed the marketplace success that they, in fact, did. If the invention were obvious, someone else would have introduced a product incorporating the patented features earlier.
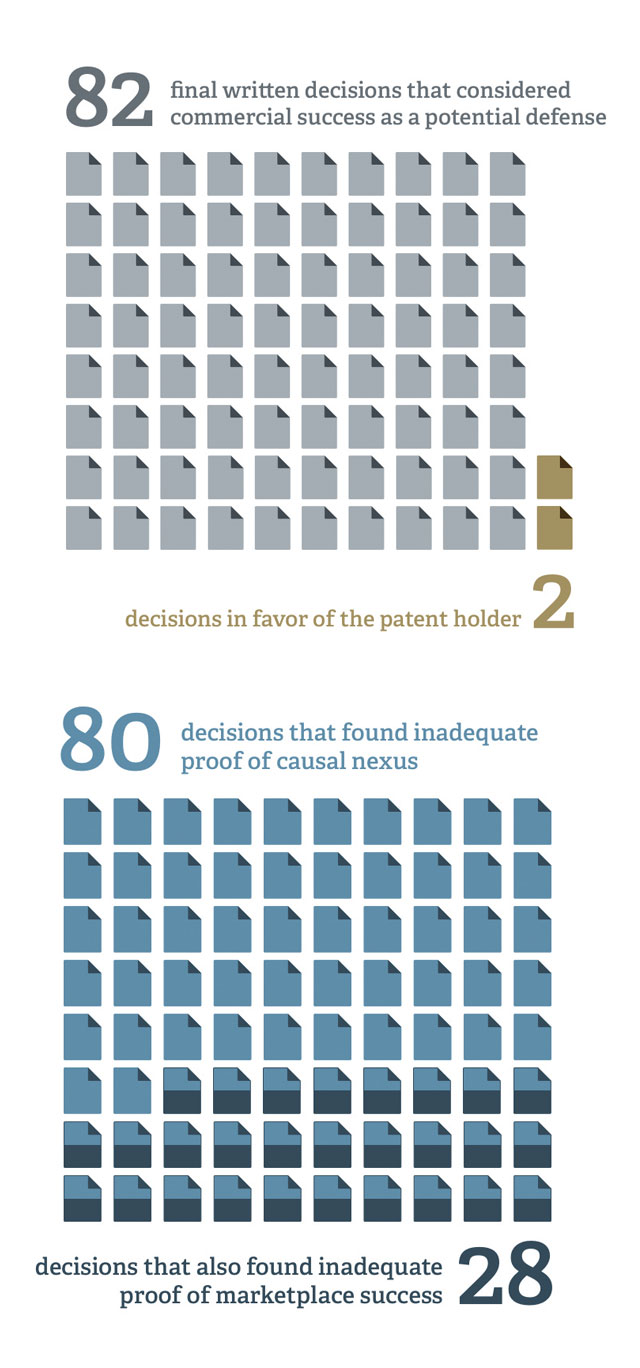
Yet, patent owners rarely have been successful at the PTAB in invoking this defense. In 82 final written decisions in IPR proceedings (through June 2015) that considered commercial success as a potential defense to patentability, the patent owner prevailed only twice. The reasons behind patent owners’ lack of success and the types of economic evidence that appear to be required for a showing of commercial success may be seen in the PTAB decisions themselves, as well as in decades of litigation in US federal district courts.
An assessment of commercial success entails a two-part analysis. First, there must be proof that the products that embody the invention have been successful in the marketplace. That is, there must be proof of marketplace success. Although neither the law nor economics provides a clear and clean definition of “success,” the PTAB and district courts appear to require that the success of the practicing products be evaluated in both absolute and relative terms. Often, the latter is accomplished by assessing the “market share” captured by the patented products and evaluating the significance of that “share” based on factors such as the number of competing products and the timing of entry into the business. The definition of competing products is usually critical in such an inquiry.
The second step in evaluating commercial success is to assess whether there is a causal nexus between the marketplace success of the products embodying the patent and the advantages of the claimed invention. As in the case of “success,” here too, neither the law nor economics provides a clear and clean definition of “causal nexus.” A causal nexus inquiry typically requires an identification of the specific features/advantages enabled by the invention, as well as an assessment of the relative importance to the marketplace of the patent’s features/advantages. Perhaps setting the bar higher than it has been set in federal court, the PTAB often has found it necessary for the patent owner to show that the product’s success is not largely owing to other features and capabilities of the product, as well as non-product characteristics of the manufacturer.
Patent owners’ failure in more than 95 percent of the written decisions that consider commercial success has been due sometimes to inadequate proof of marketplace success (which was explicit in 28 of the decisions) and often to inadequate proof of causal nexus (explicitly cited in 80 of the decisions). In short, products that practice the patent must be shown to be marketplace successes in both absolute and relative terms. And the success must be shown to be caused, in large part, by the tangible features/advantages taught by the patent. Presumptions of success or causality will not rule the day. Economic evidence must be considered carefully and presented thoughtfully. ■
-
Case in Point
ImmunoGen Patent Upheld in Victory at the PTAB
The Patent Trial and Appeal Board (PTAB) recently ruled in favor of ImmunoGen, Inc., denying Phigenix, Inc.’s petition that the claims of an ImmunoGen patent covering its breast cancer treatment, Kadcyla, were unpatentable. An Analysis Group team led by Managing Principal John Jarosz was retained by counsel on behalf of ImmunoGen to conduct an assessment of the commercial success of Kadcyla. Kadcyla is an anti-cancer therapy composed of an antibody and toxin that Phigenix contended would have been obvious over prior art references. Since the creation of the PTAB in 2012, which established new processes for post-grant and inter partes patent reviews, many US patents have been invalidated. Patent owners have had particular difficulty demonstrating commercial success, which requires an assessment of the marketplace success of the patent-practicing product and a showing of a sufficient causal nexus between marketplace success of the patented product and the claimed advantages of the patent. In its decision, the PTAB cited Mr. Jarosz’s expert testimony and analysis, which drew on revenue and prescription data, as well as marketing and promotional efforts, to provide evidence of the commercial success of Kadcyla.
-
Adapted from “Assessing Commercial Success at the U.S. Patent Trial and Appeal Board,” published in International In-house Counsel Journal, Vol. 8, No. 32, Summer 2015, 1.
John Jarosz is a Managing Principal in Analysis Group’s Washington, DC, office. Robert Vigil is a Principal in Analysis Group’s Washington, DC, office.