-
Challenges and Opportunities: Suspicious Order Monitoring of Prescription Opioids
The Suspicious Order Monitoring (SOM) requirement has become an important weapon in battling the opioid crisis in the United States.
First embodied in the 1970 Controlled Substances Act, the Drug Enforcement Agency’s (DEA’s) SOM requirement obliges any registered distributor of opioids to “design and operate a system to disclose … suspicious orders of controlled substances.” Though the agency has provided little explicit guidance as to the meaning of this requirement, it nevertheless has set a stringent standard for enforcement.
This became clear when the DEA revoked the controlled substance license of Masters Pharmaceutical for failure to comply with the SOM requirement. Like other distributors, Masters used a statistical algorithm to screen and flag orders as potentially suspicious. Flagged orders were reviewed manually for reporting to the DEA. The agency’s decision, upheld by the DC Circuit Court of Appeals, turned in part on whether Masters was using all available data and analytics to report the right orders.
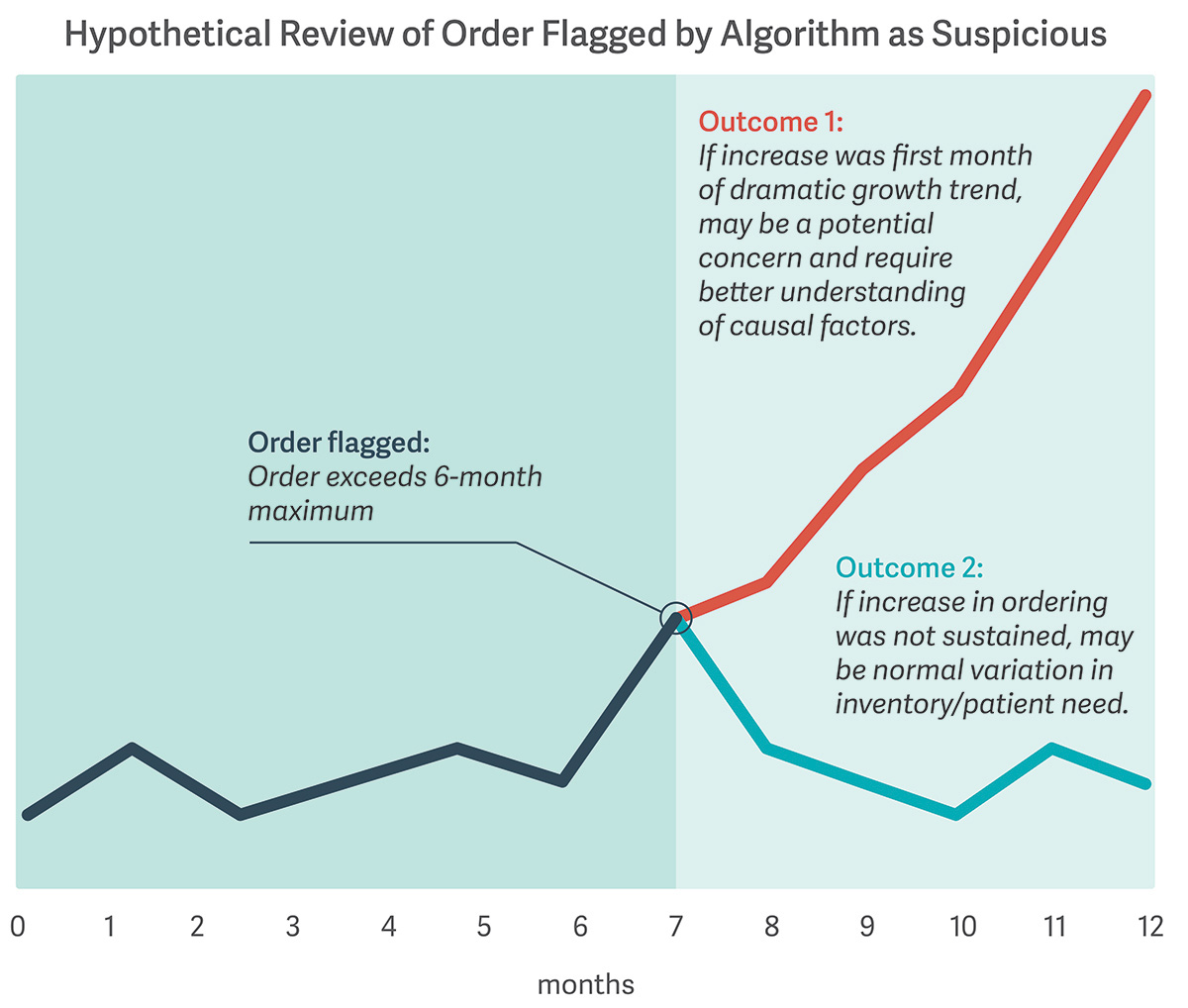
The Masters case leaves some key questions unresolved. Are distributors required to obtain additional data beyond their own to uncover potentially suspicious orders? How can they make the appropriate decision with the information available to them? (See illustrative figure.)
Nor are the challenges confined to distributors. Recent investigations have targeted manufacturers for enforcement of SOM requirements as well. Unfortunately, each member of the pharmaceutical distribution chain that has a regulatory requirement to prevent diversion of controlled substances – manufacturer, distributor, pharmacy, and provider – is limited by the incomplete scope of the data available for it to observe.
The Masters decision put forward a requirement to “use the most accurate information available” for SOM. What constitutes “available,” however, is not straightforward. With SOM being featured as a critical plank in the DEA’s approach to countering the opioid crisis, there will no doubt be increased effort to meet these requirements. Effort alone, however, does not guarantee compliance, and the uncertainty over how recent settlements should be interpreted, as well as the difficulties in obtaining and employing relevant and timely data, present substantial challenges. ■
Crystal Pike, Managing Principal
Kenneth Weinstein, Managing Principal