-
Causation in Product Liability: Taking a New Look
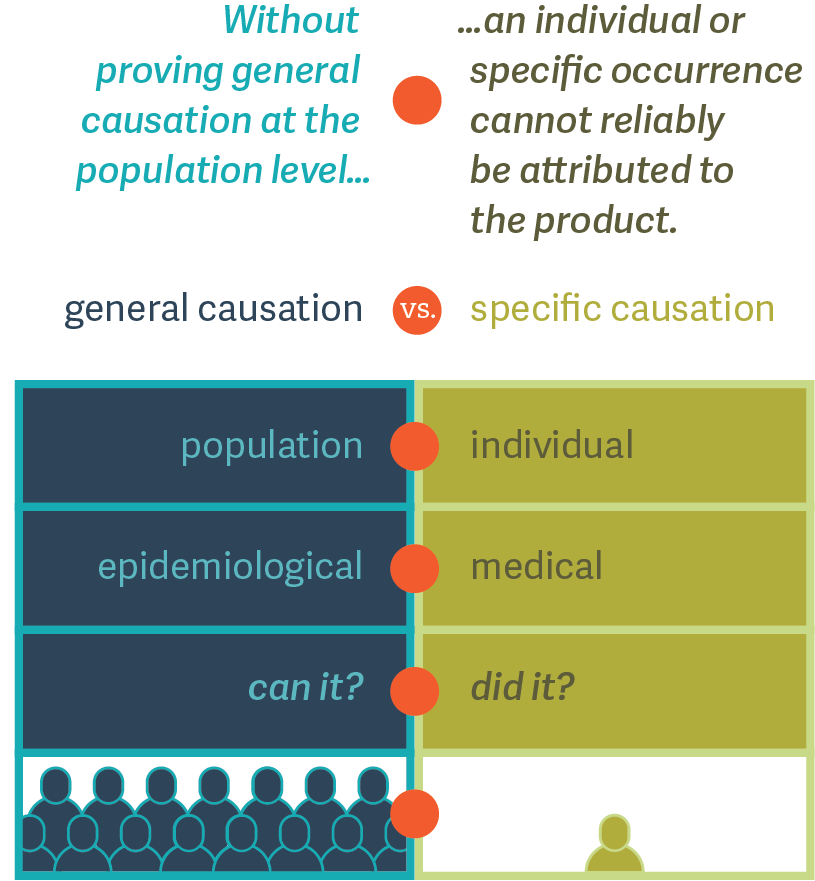
Proof of general causation can be an important precursor to establishing specific causation.
Product liability litigation for pharmaceuticals and medical devices often relies on physician testimony to establish causality for a plaintiff. In such cases, plaintiffs, defendants, jurists, and jurors alike focus on the question, “Did the drug (or device) cause a particular side effect for this person?” However, as Analysis Group Managing Principal Mei Sheng Duh points out, inferring a definite causal relationship based on any individual patient experience is unlikely to pass basic thresholds of scientific reliability.
Dr. Duh suggests that the causal relationship between a drug and an observed adverse event should be assessed at two different levels: (1) general and (2) specific. (See figure.) First, epidemiologists can test the association between a drug and a particular adverse event at the population level. This is often referred to as “general causation.” Following that, physicians may look at a particular patient’s medical history to determine whether an adverse event in that single individual is causally related to the use of the drug. This is often referred to as “specific causation.”
As Dr. Duh explains, at the population level, the aim of causality assessment is to answer the question, “Can it?” – that is, is it possible that the given drug could cause a particular adverse event in anyone? At the individual level, however, causality assessment answers the question, “Did it?” – did the drug given to a particular individual cause the particular adverse event? The first question is the realm of the epidemiologist, while the second is the realm of the physician.
For example, in a recent product liability matter, a team from Analysis Group led by Dr. Duh, Principal Brian Ellman, and Analysis Group affiliate Professor Lee-Jen Wei, of Harvard University’s Department of Biostatistics, used a population-level approach to show that, in general, exposure to the at-issue product did not increase the risk of cardiopulmonary arrest while undergoing hemodialysis, as was alleged. Instead, they showed that other factors were likely to explain such events.
Without first answering the “can it” question, answering the “did it” question could lead to the fallacy that an idiosyncratic association based on one individual is indicative of a causal relationship. Hence, litigators should not lose sight of the foundational importance of the “Can it?” question in product liability lawsuits. ■
Mei Sheng Duh, Managing Principal
Brian Ellman, Principal