-
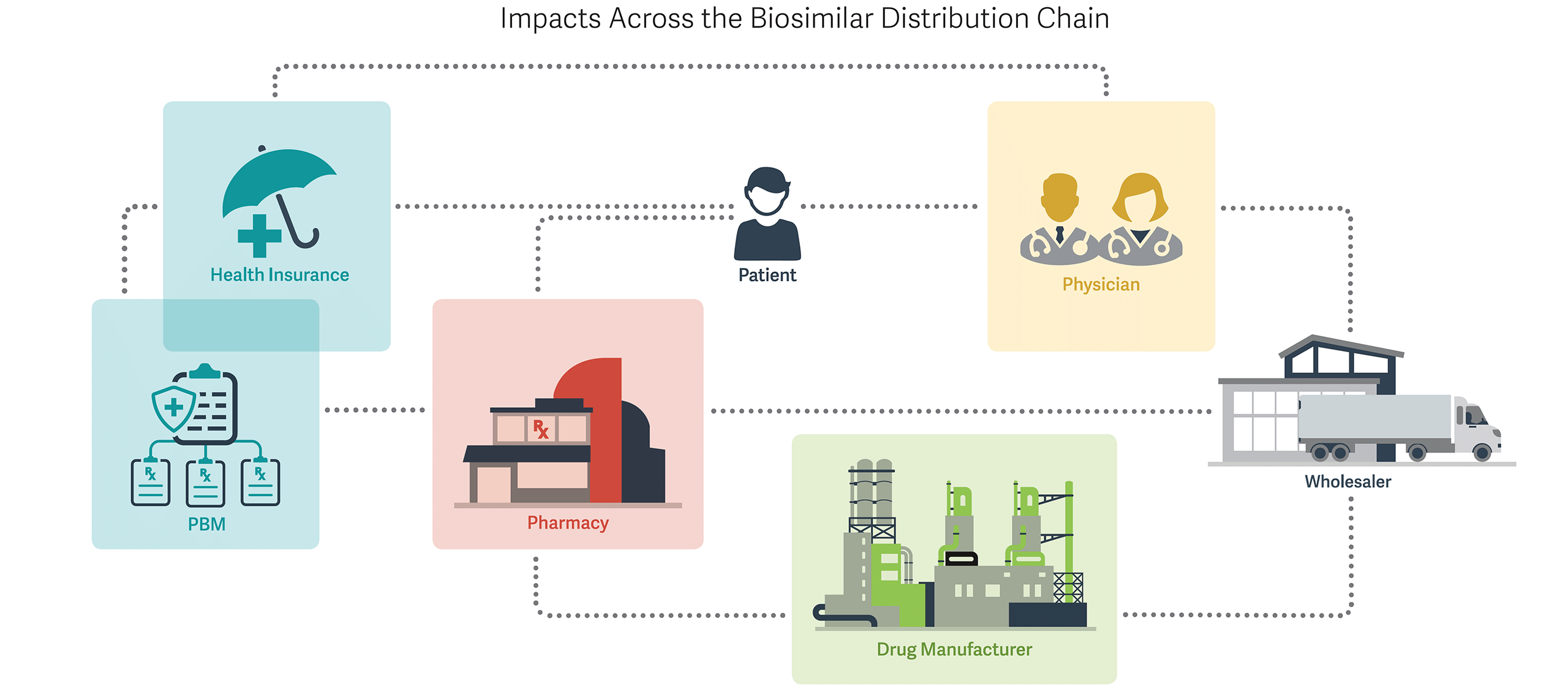
Why Biosimilar Introduction May Be Different
PBMs and Health Insurers - Customized rebating strategies for both brand biologic and biosimilar manufacturers
- Emphasis on efficacy and safety findings for individual biosimilar products, due to variability from reference biologic
Pharmacies - Modest influence of automatic substitution, as few biosimilars may receive an interchangeability rating due to scientific challenges, uncertainty, and regulatory costs
Drug Manufacturers - Marketing of biosimilars and promotion of individual characteristics rather than a generic strategy of pure price competition
- Vertical contracting and rebating with pharmacy benefit managers (PBMs) and others for preferred access
- Post-marketing trials on safety and efficacy to increase physician uptake
Physicians - Need for additional real-world evidence on efficacy and safety of individual biosimilars, and importance of individual physician experience
- Reimbursement considerations due to “buy and bill” nature of many physician-administered biologics
Biologic drugs comprised seven of the ten highest-revenue drugs worldwide in 2016.
Pharmaceutical companies are developing biosimilar versions of these lucrative products, and to date seven biosimilars have been approved by the US Food and Drug Administration (FDA) for five biologic drugs.
However, while biosimilars are the biologic drug corollary to generic drugs, they have important differences from traditional generics.
Biosimilars and their branded biologic counterparts are complex, organism-based medications that are “grown” rather than chemically manufactured. This leads to more complexity and variability in production processes and in the biosimilar products themselves.
These circumstances, in turn, create a cascade of impacts throughout the distribution chain, affecting everything from production costs and marketing strategies, to regulatory oversight of efficacy and competition, to physician uptake, to pharmacy fulfillment and payer reimbursement considerations.
Understanding the chain of impacts is critical to understanding the economic implications of biosimilar competition and the unique issues raised in prospective intellectual property, antitrust, product liability, and other litigation. ■
For more information, please contact: Richard A. Mortimer, Managing Principal