-
Protecting Access, Encouraging Innovation: A Discussion About Drug Pricing
Drug prices dominate the news in a way few other health care topics do.
Spending on prescription drugs, which accounts for about 10–15% of annual health care spending, is the fastest-growing part of most commercial insurance plans, and has become a highly visible symbol of the challenges of controlling overall health care spending in the United States. What tools might help make pharmaceuticals more accessible to people who need them? Does drug pricing adequately reflect the necessary capital costs of bringing new therapies to market? What role do negotiated rebates play in this system? Can increased competition play a role in controlling prices?
These and other topics were the subject of a lively debate about drug pricing at Analysis Group’s annual Law & Economics Symposium on life sciences topics. Moderated by Managing Principal Noam Kirson, the expert panel comprised Jennifer Bryant of PhRMA, Rena Conti of Boston University, David Cutler of Harvard University, Craig Garthwaite of Northwestern University, and former FDA Commissioner Scott Gottlieb. Below we briefly recap the three main topics the panelists discussed.
-
Balancing Priorities in Drug Pricing
Noam Kirson, Managing Principal
This panel was a valuable opportunity to bring together a group of leading researchers and experts to discuss current drug pricing issues and assess various policy options. Though the panel covered a range of different topics, they all revolved around a central tension in this area: the need to balance budgetary impact and affordability with maintaining incentives for research and development of future therapies. In other words, how can drugs be priced and financed so that people who need them have access to them while maintaining a market structure that encourages the development of future breakthroughs?
The ideas that the panelists suggested, questioned, and debated represent some of the mechanisms that stakeholders have proposed to address this basic tension. None of these policies is likely to provide an easy solution, but rigorous and open debates such as the one this panel engaged in will be key to charting a way forward in the future.
-
Rebates: Who Reaps the Benefits?
Much of the conversation centered on the topic of rebates – confidential discounts off the list price negotiated by either insurers or pharmacy benefit managers (PBMs) with drug manufacturers in exchange for placing those drugs on the insurer’s formulary of covered medications. Panelists debated whether these discounts – which are not directly passed through to plan participants – should be eliminated as part of a strategy for reducing out-of-pocket costs.
The panel also discussed recent calls to allow Medicare to directly negotiate drug prices.
Price Benchmarks: Looking to the World
One frequently discussed way of controlling drug prices is the use of an international price index (IPI) benchmarking system. The goal of such a model would be to reduce the price paid by Medicare for a drug by setting its target price closer to what other nations pay.
Some reservations about the efficacy and the effects of the system were raised by panelists, such as that it would be detrimental to competition and would significantly reduce investment in future therapies. And, as a recent Analysis Group article points out, even in European drug markets, where benchmark comparisons are mandatory in certain situations, determining the appropriate target prices is a highly complex undertaking.1
New Pricing Models: Netflix, Anyone?
The panel addressed whether innovative pricing methods might help ensure access to higher-priced therapies. Such models have already been proposed and debated for gene therapies – including, for example, long-term financing plans for expensive, “one and done” treatments, and agreements that link payments to therapeutic outcomes.2
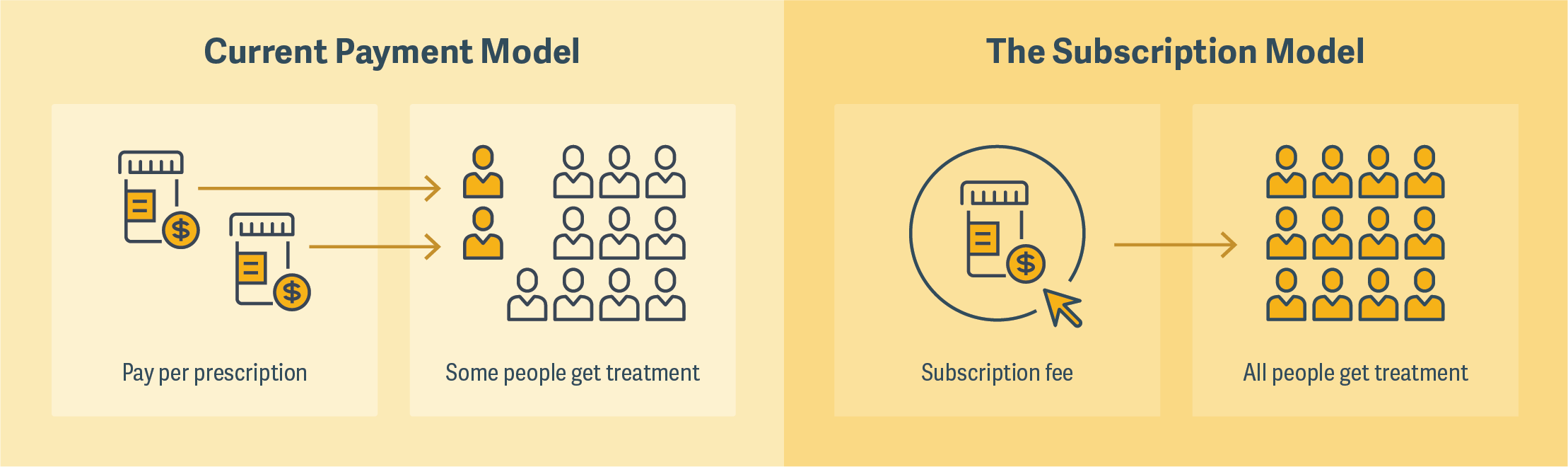
One that was much discussed by panelists was the so-called “Netflix model,” a subscription model that Louisiana has recently employed to cover hepatitis C treatments for its Medicaid and prison populations. The state agreed to pay a drug manufacturer a fixed amount and, in return, receive unlimited doses of the medication. (See figure.) The program was described by some panelists as a “win-win” because it would greatly expand the number of people who would have access to a particular treatment and give drug manufacturers certainty about revenue.
There was some discussion, however, about the conditions under which such a model could be successful, and whether it could be broadly applicable. There were also reservations expressed about the market signals that would be sent by lowering an already cost-effective price for a drug, which might in turn contribute to underinvestment by venture capital in the pharmaceutical market. ■
Click on the graphic below to view an animated version
-
1. “Economics of Excessive Pricing: An Application to the Pharmaceutical Industry” by Claudio Calcagno, Antoine Chapsal, and Joshua White, Journal of European Competition Law & Practice, March 2019.
2. “Are Payers Ready to Address the Financial Challenges Associated with Gene Therapy?” by Michael Ciarametaro, Genia Long, Michaela Johnson, Noam Kirson, and Robert W. Dubois, Health Affairs Blog, June 28, 2018.