-
Breakthrough Therapy May Increase Transplant Access and Improve Outcomes for Patients with Blood Cancer Across Racial and Ethnic Groups
A new donor source could help improve transplant outcomes for patients with certain blood cancers if it is available to those who are underrepresented in donor registries.
Last year, the US Food and Drug Administration (FDA) approved a modified cell therapy derived from umbilical cord blood as a new donor source for transplant patients with some blood cancers. That therapy, omidubicel-onlv, is the first of its kind and has been shown in clinical trials to improve immune recovery and decrease infection rates in patients who undergo allogeneic hematopoietic cell transplantation (allo-HCT), as compared to standard cord blood.
“[U]se of omidubicel-onlv in a large, diverse patient population could lead to improvements in transplant use and clinical outcomes, with the greatest benefits seen among racial and ethnic minorities.”– Khera, et al., 2024
Although allo-HCT is widely accepted as curative for several diseases, many patients – especially those who belong to racial and ethnic minority groups – may have trouble finding matching/matched donors from a registry to initiate their journey toward transplantation. Since cord blood has less matching criteria than bone marrow, omidubicel-onlv has the potential to reduce disparities in access to allo-HCT across racial and ethnic groups because it widens the pool of available donors and lessens the chance of post-transplant rejection and infection.
In our study, we used a decision-tree model1 to project how the availability of omidubicel-onlv could help address health inequities among patients eligible for allo-HCT. Below, we summarize two major findings.
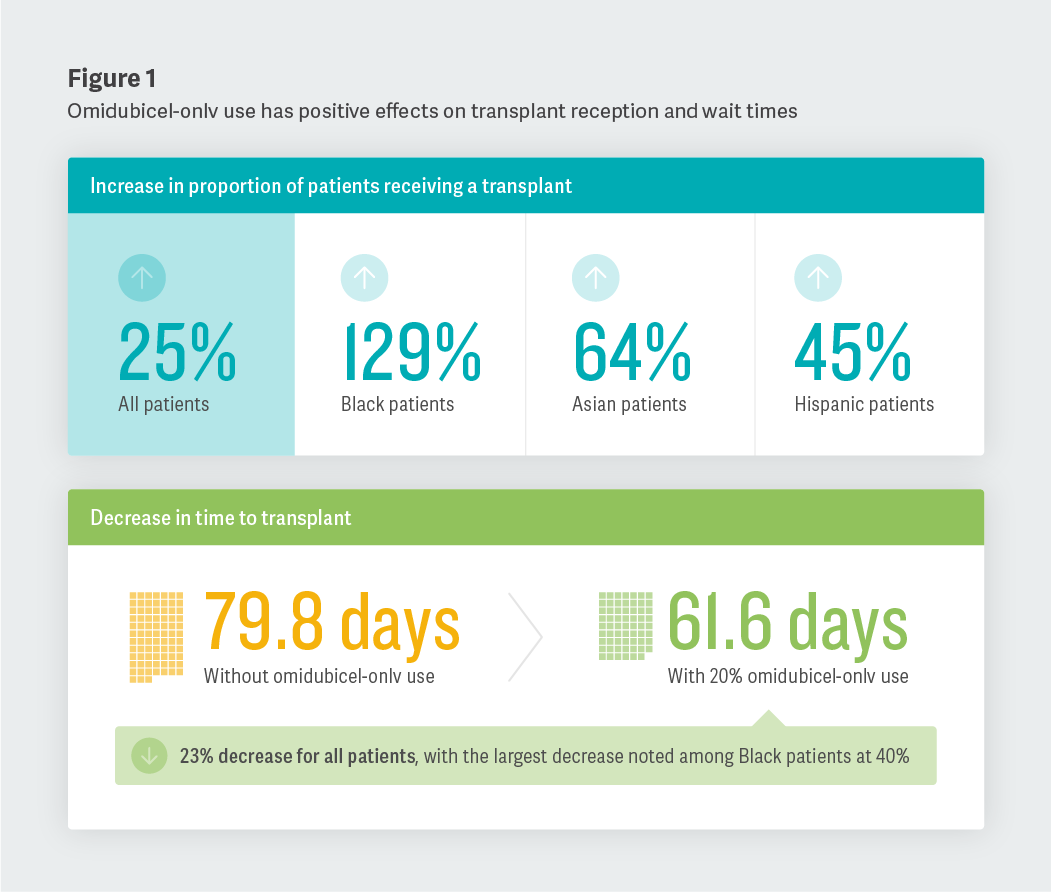
Increasing Transplant Likelihood and Decreasing Time to Procedure
First, we found that increasing the use of omidubicel-onlv across a population of eligible transplant patients could increase the proportion of patients who receive allo-HCT and decrease the time that patients spend waiting for a transplant in that population. For example, when comparing a patient population that documents 0% use of omidubicel-onlv with one that reports 20% use, we saw an increase in the proportion of patients receiving a transplant. This increase was most notable among Black, Asian, and Hispanic patients. We also observed a significant decrease in time to transplant across all patients, with the largest decrease noted among Black patients.
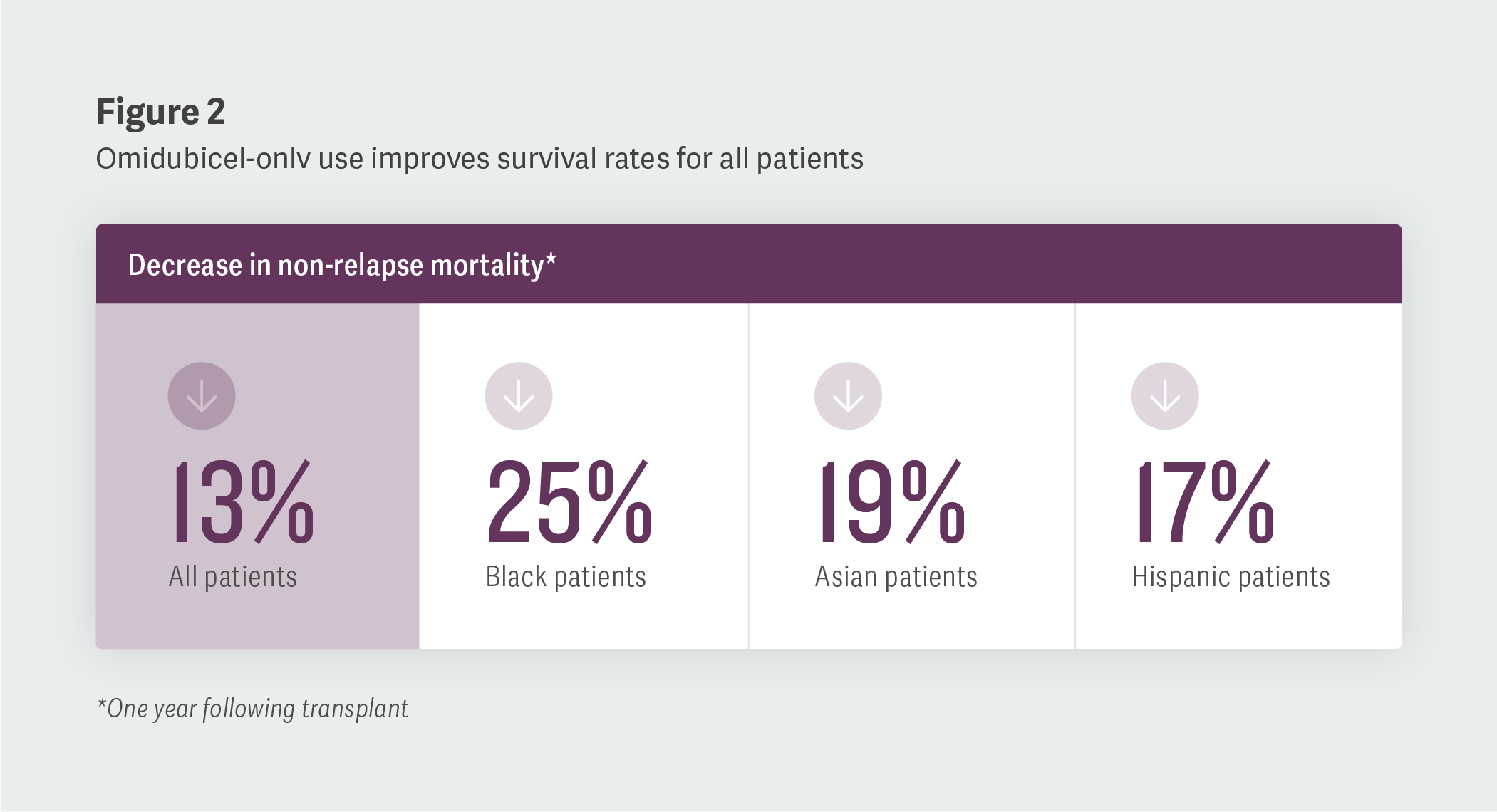
Improving Non-Relapse Mortality and Overall Survival
Second, we noted that an increase in omidubicel-onlv use was correlated with a decrease in non-relapse mortality (patients who die without experiencing a relapse in their condition) and an increase in overall survival, one year removed from transplantation. Specifically, we observed reductions in non-relapse mortality in the overall cohort studied, with the largest decreases among Black, Asian, and Hispanic patients. We also saw increases in overall survival (one year following transplant) of 3% in the overall population, with improvements ranging from 3% among white patients to 5% among Black patients.
Conclusion
Our study indicates that a broad introduction of omidubicel-onlv as a new donor source could increase allo-HCT access and improve outcomes among eligible transplant patients. Further research, including real-world studies based on data collected from electronic medical records or patient charts, could help shed light on omidubicel-onlv uptake in clinical practice. We hope that this study can be used to increase awareness about the effectiveness of omidubicel-onlv, especially among patients who are underrepresented in donor registries. ■
James Signorovitch, Managing Principal
Yan Song, Vice President
Marie Louise Edwards, ManagerEndnotes
- The estimates produced by this model were founded on assumed levels of omidubicel-onlv adoption and the application of clinical trial outcomes to real-world settings. The clinical data used was derived from the most robust evidence available in the literature at the time of the analysis.
Adapted from “Projected Impact of Omidubicel-onlv on Racial/Ethnic Disparities in Allogeneic Hematopoietic Cell Transplantation (Allo-HCT) Outcomes in Hematologic Malignancies,” Advances in Therapy (2024), coauthored by Nandita Khera (Mayo Clinic); Marie Louise Edwards, Yan Song, Rochelle Sun, and James Signorovitch (Analysis Group); Rocio Manghani, Heayoung Shin, Ronit Simantov, and Smitha Sivaraman (Gamida Cell); and Usama Gergis (Thomas Jefferson University).
This feature was published in September 2024.