-
Which Problem to Solve? Assessing the Complexities of Drug Pricing in the US
Lower drug prices? Popular. A strategy everyone can agree on? Elusive.
Drug prices regularly command public attention, and proposals to lower them are a regular feature of the US public policy landscape. Re-examining and potentially lowering the costs of some drugs has been a topic the Biden administration has put under renewed scrutiny.
But the US pharmaceutical pricing ecosystem is complex, with a large number of participants, regulations, and market incentives. Within this vast pipeline, it can be difficult to achieve agreement on what’s causing the problem, let alone how to solve it.
Gaining some clarity around these issues was the goal of Analysis Group’s first 2022 Law & Economics Symposium webinar. Moderated by Noam Kirson, the webinar brought together three distinguished participants – Anna Kaltenboeck, Jeffrey Handwerker, and Joshua Cohen – to examine the workings of the American pharmaceutical market and discuss the ongoing debate over what solutions are likely candidates for lowering consumer prices.
What Happens Next? Value Assessment, Drug Pricing Policy, and the R&D Pipeline
June 8, 2022 | 12–1 p.m. ET
A Focus on Policy
Ms. Kaltenboeck began the webinar with an overview of current policy efforts to lower drug prices. She noted that a disproportionate amount of the approximately $500 billion the US spends annually on prescription drugs is driven by specialty branded drugs that only account for between 2% and 5% of total prescriptions. She submitted that the reconciliation bill currently under consideration in Congress would address three problems related to the high prices for those drugs: financial toxicity (by capping out-of-pocket spending for Medicare Part D), consolidation in the supply chain (by redesigning the way Part D pays for drugs), and pricing power (by empowering Medicare to negotiate prices).
Mapping the Terrain
Mr. Handwerker pointed out that the entire question of a drug’s “price” is much more complicated than it might initially seem. In a diagram, he detailed the system of drug manufacturers, wholesalers, pharmacies, pharmacy benefit managers (PBMs), and insurance companies that constitutes this network.
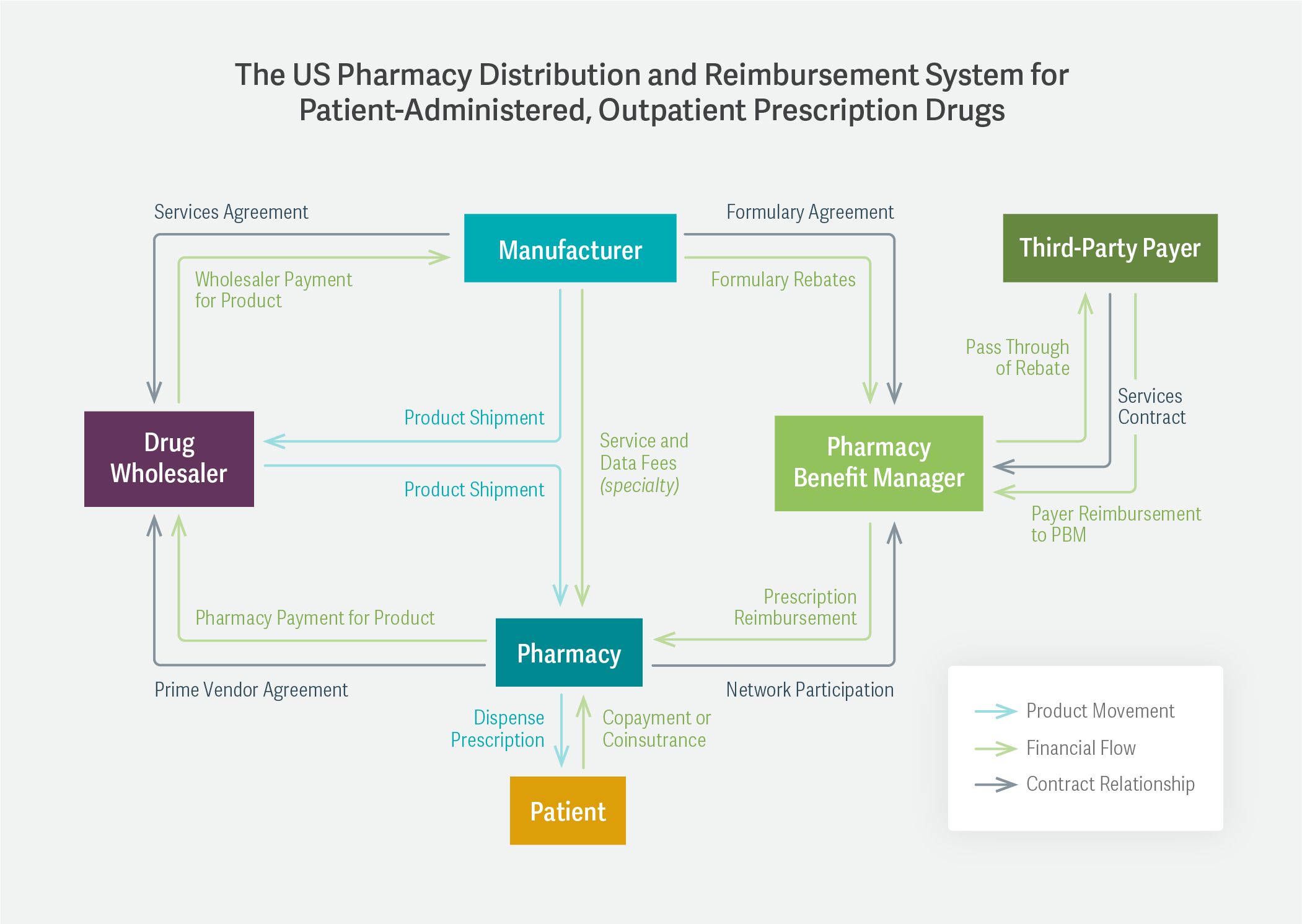
The complexity of the relationships among these participants – involving not only wholesale and retail costs, but also rebates, formulary discounts, and copayments – results, in many cases, in a lack of transparency around how the wholesale acquisition cost (set by the manufacturer) eventually translates into the patient’s out-of-pocket cost. Mr. Handwerker also said that it was important for federal agencies to examine whether ongoing vertical integration in the health care space has harmed competition.
Aligning on Value
An important issue when considering drug pricing policy is balancing the clinical benefits against the prices needed to incentivize drug manufacturers’ investment in risky innovation. One approach to evaluating drug prices in light of this issue is value-based pricing, which was the focus of Professor Cohen’s presentation. How to assess a drug’s value is, of course, a crucial question, and Professor Cohen detailed the ways in which the Institute for Clinical and Economic Review (ICER) and other health technology assessment (HTA) organizations do so by using measures such as the quality-adjusted life year (QALY) and the cost per added cured individual. How to use these and other measures to properly assess value and to balance access and innovation remains a critical topic.
Pondering Solutions
A series of interesting exchanges followed. Dr. Kirson homed in on whether there would need to be a sacrifice on the innovation side in exchange for lower prices. Ms. Kaltenboeck and Mr. Handwerker discussed whether capping prices for older drugs that have been on the market for a considerable length of time could improve the situation, or whether it was preferable for drug companies to use those profits to fuel further innovation.
The panel also discussed whether HTA review of the kind provided by ICER could or should be part of the pricing equation, especially at the level of government policy. Professor Cohen pointed out some of the limitations of standard HTA metrics, and advocated for a number of organizations conducting such analyses and functioning as checks on each another. Mr. Handwerker and Ms. Kaltenboeck discussed whether the recent US Food and Drug Administration (FDA) approval of the Alzheimer’s drug Aduhelm was proof that the decision-making process around Medicare coverage was functioning properly.
No easy answers emerged from the discussion, but the diversity of opinion on the panel offered a helpful introduction to the issues central to this debate. ■
This feature was published in August 2022.