Analysis Group Research Improves the Quality of Real-World Data for Blood Disease Treatment in China
February 11, 2022
The use of real-world data (RWD) and real-world evidence (RWE) has transformed health care research, improving both clinical decision making and oversight of drug safety and effectiveness. Many times, however, the lack of high-quality research-grade data regarding patient treatment regimes and outcomes has hindered the use of RWE. In China, for example, the fragmentary data available on treatment regimen and patient response have impeded researchers’ ability to use RWE to advance knowledge of blood diseases. To overcome this challenge, Analysis Group collaborated with the Institute of Hematology & Blood Diseases Hospital (IHBDH) to establish the National Longitudinal Cohort of Hematological Diseases in China (NICHE), a registry of high-quality RWD from tens of thousands of patients across the country.
Major strides have been made in improving the usability of RWD through the NICHE initiative. An important milestone in this development is a methodology study to improve the quality of research-grade RWD for acute myeloid leukemia (AML). While guidelines for treatment regimens for AML are well known, there is limited knowledge of how patients in China are treated in a real-world setting. An Analysis Group team led by Managing Principal Eric Q. Wu and Manager Jia Zhong created algorithms that helped researchers to overcome this gap by using RWE to retrospectively create complex AML patient journeys in China.
In the study, researchers selected 4,980 NICHE patients with AML, and assembled RWD from multiple sources, including hospital information systems (HIS), electronic medical records (EMRs), genetic testing panels, and picture archiving and communication systems. The team synthesized recommendations from AML treatment guidelines and clinical consensus to create a model of AML management through algorithms capable of reconstructing lines of therapy, multi-agent treatment regimens, and treatment response. The model yielded high sensitivity (91.7%) and specificity (97.6%) against clinical evaluation by IHBDH hematologists, using a validation sample. The validated algorithms were then implemented through the Huashu Hematology Medical Information Management System (HHMS).
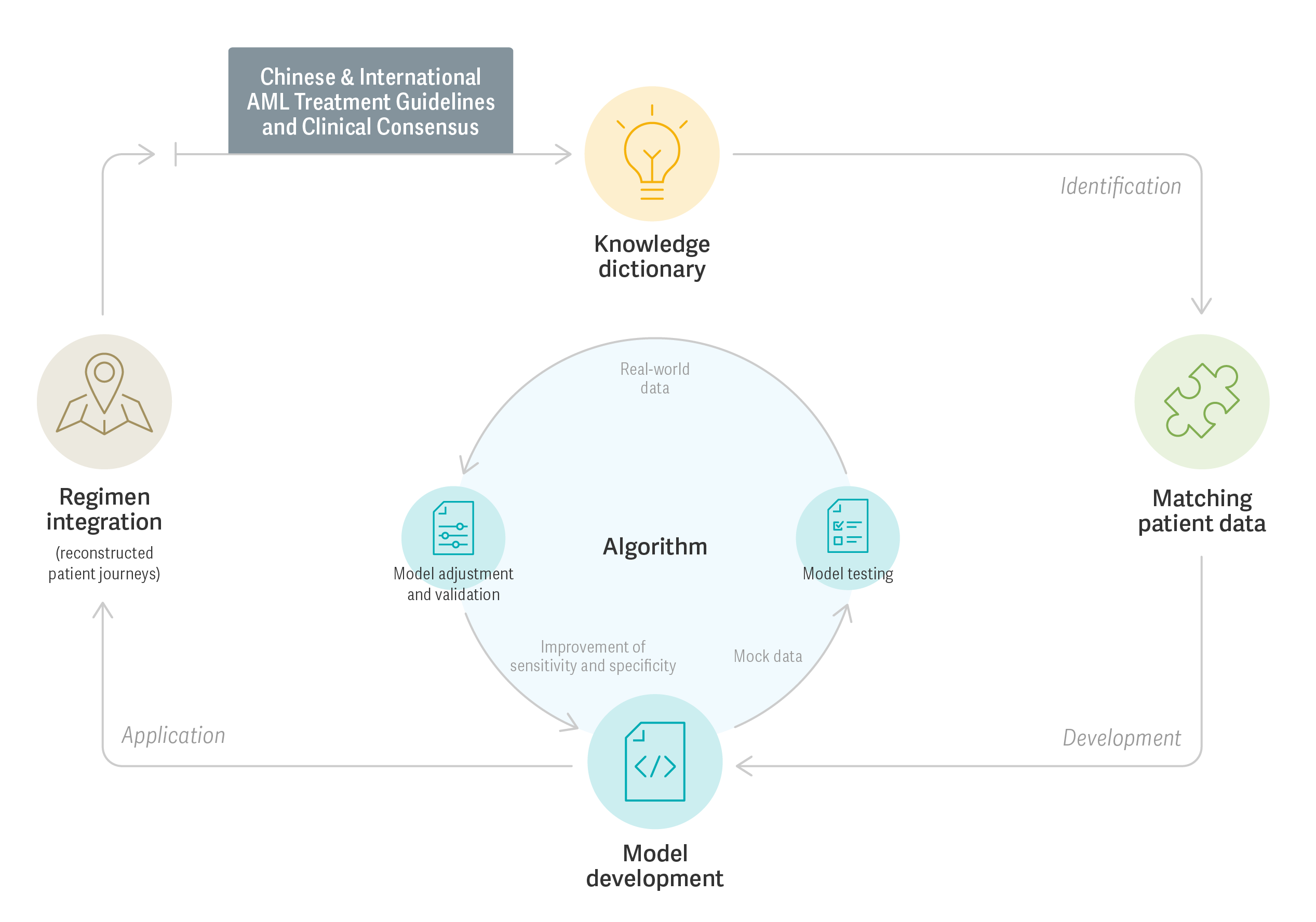
In carrying out this project – the details of which were presented at the Ninth Southern China Annual Congress on Pharmacoeconomics – the team was able to rectify inadequacies in the NICHE data, making them suitable for further analysis on treatment patterns, comparative effectiveness, and safety and enhancing the understanding of the AML patient management in China.
